CSSD Products Suppliers in Mumbai
In contemporary hospitals and healthcare settings, the Central Sterile Supply Department (CSSD) plays a key role in providing safety for the patient and controlling infections. Medical sterilization processes depend greatly on the Central Sterile Supply Department responsible for the decontamination, cleaning, sterilization, packaging, and distribution of medical equipment and surgical instruments. A properly equipped and managed CSSD is paramount in maintaining clean and hygienic standards to guarantee the seamless operation of the clinical services.
CSSD products are vital. Equipment of poor quality has a real impact on infection prevention and control, and regulatory requirements. If care facilities are not able to meet requirements, they may cause delays, introduce, and contaminate patient enhancing the risk of harm to their health.
This is where Hybeat is positioned to be understood. We has an understanding of high-end Unreal CSSD solutions, and they are the principal supply distributors and dealers in Mumbai, Maharashtra. We provides hospitals and care facilities with a comprehensive range of solid, sanitary, and purpose-built CSSD products to enable the development of sterile environments that conform to local Indian and global healthcare regulations. Hybeat is recognised as the top supply distributor, dealer in Mumbai, Maharashtra, validated by healthcare professionals wanting a product that is high-performance and reliable, offering involvement and longevity.
Understanding CSSD: What It Is and Why It Matters
The Central Sterile Supply Department, or CSSD, is one aspect of infection control strategies in health care environments. The central sterile supply department operates as a central process point for the processing, decontamination, storage, and sterilization of surgical instruments, medical equipment, and other reusable hospital materials. If sterilization is done improperly, nosocomial infections can be caused, which puts both patients and practitioners at risk.
A competent central sterile supply department normally has four distinct areas: a reception area for collecting used instruments, a cleaning and disinfecting area for pre-treatment, a sterilization area utilizing pressure steam or chemicals, and a storage area for sterile items waiting for re-distribution. All four areas must be logically laid out and function in unison from a workflow and contamination standpoint.
The nature of the CSSD products being used obviously affects the performance and safety of the CSSD products. Therefore, it is important that high-quality stainless steel trolleys, washer disinfectors, containers, modular storage systems, etc., be selected to maintain sterility, ease of cleaning, and longevity.
We are the leading supplier, distributor, and dealer of CSSD products in Mumbai, Maharashtra. We aims to provide robust, hygienic, and ergonomically designed CSSD products.
Key CSSD Products Offered by Hybeat
we offers a wide variety of high-performance CSSD products designed for the precise demands of hospitals and laboratories for effective and hygienic sterilization methods. Hybeat is the premier Independent supplier, distributor, and dealer in Mumbai, Maharashtra, offering total solutions with durability, accuracy, and medical standards.
The core product line provides stainless steel equipment, which includes but is not limited to trolleys, work tables, instrument racks, and loading systems. This equipment is manufactured from corrosion-resistant SS304 and SS316 grades to be durable for longer periods, as well as allowing for easy sanitation capabilities. With their smooth welds, ergonomic designs, and mobility options, these units provide sterile operations while maintaining workflow.
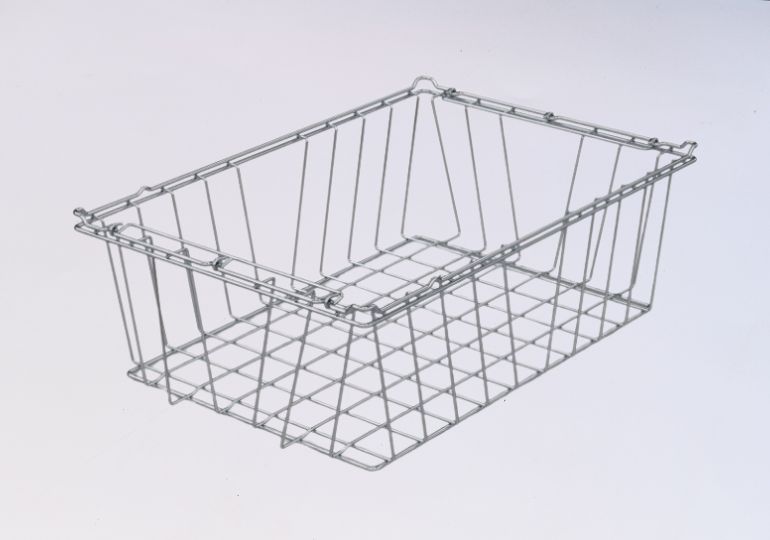
We provides sterilization containers, baskets, and trays that are necessary to safely hold instruments both during and after the sterilization process. These products are made from medical-grade materials, can be made in multiple sizes, and are introduced as stackable and reusable to provide a safe, hygienic, and organized environment throughout the sterilization process.
We also offers automated cleaning and drying equipment, including instrument washers and drying cabinets for easy re-processing of surgical instruments.
Why Quality CSSD Products are Critical for Hospital Efficiency?
The products utilized in a Central Sterile Supply Department are vital in providing a safe hospital environment, protecting both patients and preventing infection. Patient safety and well-being will be compromised when surgical instruments and equipment are not correctly sterilized, which could potentially lead to hospital-acquired infections (HAIs) that not only negatively influence the outcome of patients but also increase risk management. The use of high-quality components is essential for Central Sterile Supply Department work because it will ensure that thorough decontamination processes are followed, safe handling practices are adhered to, and sterile assurance is applied in low-risk quality measures to aid risk mitigation for both patients and staff.
Ensuring effective workflow continuity in sterilization departments is equally important. Ergonomically developed trolleys, racks, and washer-disinfectors make the task from reception to storage uncomplicated, ultimately reducing turnaround time for instruments and medical devices and minimizing potential human error and mistakes. The change in productivity adds seconds to the daily lives of the hospital staff in a very competitive and fast-paced healthcare environment.
To maintain accreditation and new heights in healthcare quality, hospitals must adhere to NABH guidelines and international standards. Hospitals require CSSD components to work efficiently, and expertly developed operating CSSD equipment. This is a promise to the performance.
Hybeat is readily available to deliver efficiency through best-in-class CSSD products and services.
What Sets Hybeat Apart from Other Dealers and Suppliers?
What sets Hybeat apart from all of its dealers and suppliers is simply Hybeat's commitment and extensive experience in our deliverables to the healthcare infrastructure in the Mumbai, Maharashtra area. By having such a large footprint in the local community, Hybeat has faster response and delivery times so hospitals and clinics can keep their sterilisation programs running.
We are the best positioned to provide custom CSSD layouts and product solutions that fit the specific products and processes of each facility. Hybeat provides holistic, fully integrated solutions in the range of custom services like maximising space, optimising sanitation, or sourcing interchangeable parts. Hybeat's flexibility has made it the supplier of choice for customers who want to rely on a single supplier for purchasing ease and operational simplicity.
Hybeat's pricing offers strong value along with full after-purchase support. From in-store installation to recurring maintenance, customers benefit from total support that enhances their peace of mind and extends the equipment's longevity.
All product and solution platforms meet standards for quality, safety and are built to meet strict medical compliance regulations. Products such as precision sterilization trolleys and cabinets that require corrosion resistance exhibit Hybeat's commitment to dependable and compliant products.
Proven Impact of Hybeat’s CSSD Products in Mumbai’s Healthcare Sector
Hospitals and diagnostic labs in Mumbai are noticing improvements in efficiency and cleanliness since implementing Hybeat's CSSD solutions into their central sterile supply departments. Healthcare facilities were unable to sustain consistent compliance with both NABH and international benchmark standards due to several common issues because these very same hospitals had previously experienced subpar equipment breakdowns, cluttered sterilization systems, and repeated infection control dilemmas.
Hybeat's tailor-made CSSD solution, consisting of long-lasting stainless steel carts, high volume washers, and flexible storage configuration, has addressed these problems. The ultimate design of these solutions was based on the available space, sterilization, and volume of processing, and compliance requirements specific to each facility. With this result, hospitals realized positive efficiencies in the sterilization process, improved processes, reduced contamination risks, and fewer failures due to equipment breakdowns.
Hybeat has shown that it is the leading distributor, supplier, and dealer of CSSD solutions in Mumbai, Maharashtra, by providing dependable services, a predictable supply chain, and reliable installation. In Mumbai, many private hospitals and multispecialty clinics have enlisted the hospitals to depend on Hybeat systems for infection control and to fulfill compliance obligations - it shows the increasing traction of Hybeat systems in Mumbai's health system.
Service, Support, and Installation by Hybeat
We provides a full-service solution for all CSSD equipment in health care facilities in Mumbai, Maharashtra. This service includes installation and ongoing service support. We also provides customized consulting services for all aspects of the process from inquiry to completion with a focus on designing equipment layouts that fit with the site's work processes and physical space to improve sterilization practices and reduce the space footprint for CSSD equipment.
Hybeat provides service agreements that can be tailored so that the equipment operates at optimal performance capabilities. A team of professionals is always available to provide service support, urgent equipment repair, and site support technical service to help you maximize uptime and satisfaction with compliance.
Hybeat provides localized support in Mumbai, Maharashtra for timely response and continual follow up with service engagement. Customers can be assured that Hybeat will provide a premium product, and also provide a service package that continues to provide after the goods have been commissioned and are operational. Hybeat is gaining a solid reputation for reliability and value emphasizing performance and compliance with public health standards as a leading supplier, distributor and dealer of CSSD solutions in Mumbai, Maharashtra.
Future of CSSD Products and Hybeat’s Innovation
The development of products for CSSD is evolving from new influences like automation, digital monitoring, and the next phase of the IoT. In many ways, it is changing the way our sterilization departments function, how sterilization cycles are monitored (real-time), how documentation is automated, how regulatory compliance is more efficient, and how prediction models can alert us to potential equipment failure. Hybeat is taking the charge in a fast-paced and changing landscape by creating an innovation culture and continuing to build its commitment to research and development.
Hybeat is regularly investing in the development of CSSD products for the future that provide today's health care requirements, and that align with the expected specifications of tomorrow's intelligent hospitals. New product offerings will include automated systems designed to manage sterile materials, RFID-enabled tracking of instruments, data-driven performance dashboards for sterilization, etc. Hospitals are becoming more efficient, improving accountability and advancing patient safety.
This position, as a pioneer brand from the heart of India in Mumbai, Maharashtra, Hybeat's focus is on the supply of CSSD equipment, but also approaches the market as an innovator and collaborator on technology. Hospitals and medical establishments in Mumbai trust Hybeat to deliver reliable CSSD equipment, but also to deliver on a new vision and leadership around the future of healthcare.
Conclusion
Choosing the right CSSD equipment is essential for all healthcare facilities wanting to maintain high levels of cleanliness and patient safety and for maximizing productivity. Hybeat is an excellent partner offering many quality CSSD equipment solutions that promote optimal performance, durability, and full regulatory compliance. Hybeat offers equipment solutions like sterilisation containers and stainless steel modular furniture, and high-tech cleaning and drying systems, all for an evolving hospital environment.
What sets Hybeat apart is its emphasis on trust, quality, and local knowledge. With a large footprint in Mumbai, Maharashtra, Hybeat resolves the challenges healthcare facilities in the area face with their demanding requirements, through tailored solutions with appropriate timing, service, and support. Hybeat takes care of everything for every customer, from consulting and layout planning through to installation and maintenance.
If you are a healthcare facility that needs to improve or set up an effective sterilisation department, your decision will be easy. Contact Hybeat, the leading supplier, distributor, and dealer in Mumbai, Maharashtra, to deliver reliable, innovative, and modern CSSD solutions. By working with Hybeat, you can elevate your adherence to infection control and sterilisation standards with confidence and ease.